What is acidic soil p2? This exploration delves deeper into the complexities of acidic soil, examining its causes, impacts on plants, and effective mitigation strategies. We’ll uncover the science behind soil pH, the factors influencing its acidity, and the crucial role of testing and monitoring in maintaining healthy soil for optimal plant growth.
Understanding the pH scale and how it affects nutrient availability is key. Different plants have different needs, and acidic soil can lead to deficiencies. This post will also discuss practical methods for adjusting soil pH, from using lime to alternative amendments, along with the importance of consistent monitoring.
Defining Acidic Soil pH
Understanding soil acidity is crucial for successful gardening and agriculture. Different plants thrive in different soil conditions, and knowing the pH level allows gardeners to tailor their practices to optimize plant growth. Acidic soil, while sometimes problematic, can also be beneficial for certain types of vegetation. This section dives deep into the definition of soil pH, its measurement, and the various methods for determining it.Soil pH is a measure of the acidity or alkalinity of the soil.
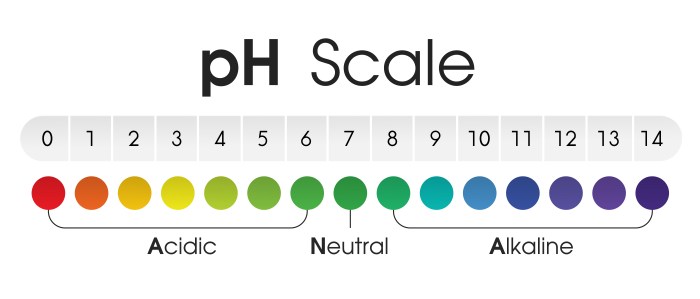
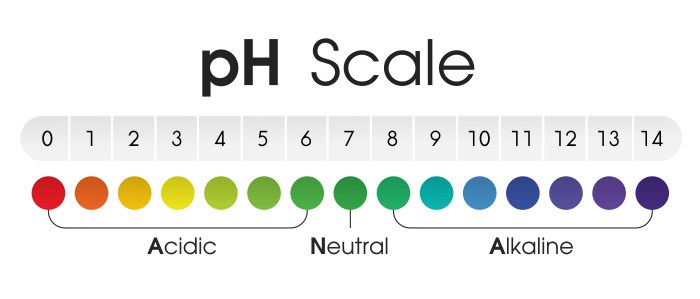
It’s a crucial factor affecting nutrient availability, microbial activity, and ultimately, plant health. The pH scale, ranging from 0 to 14, provides a way to quantify the concentration of hydrogen ions (H+) in the soil. A lower pH indicates higher acidity, while a higher pH signifies alkalinity. A neutral pH, 7, is considered neither acidic nor alkaline.
Soil pH Measurement and the pH Scale
The pH scale is logarithmic, meaning that each whole number change represents a tenfold difference in hydrogen ion concentration. For example, a soil with a pH of 6 is ten times more acidic than a soil with a pH of 7. This logarithmic nature is important to understand when interpreting soil test results. Accurate measurement of soil pH is critical for proper soil management and plant health.
Typical Acidic Soil pH Range
Soils with a pH below 7 are considered acidic. The specific range considered acidic varies depending on the context, but generally, a pH below 6.0 is considered acidic soil. Soils with a pH between 6.0 and 7.0 are considered slightly acidic to neutral. Soils with a pH above 7.0 are alkaline. Different plants have different optimal pH ranges, so knowing the specific needs of your plants is essential.
Importance of Accurate Soil pH Testing
Accurate soil pH testing is vital for several reasons. Firstly, it ensures the correct nutrients are available to plants. Different nutrients become more or less available at different pH levels. Secondly, it helps maintain optimal soil microbial activity, crucial for nutrient cycling and decomposition. Finally, it guides the selection of appropriate plants for the specific soil conditions, preventing unnecessary stress and potential plant damage.
Methods for Soil pH Testing
Several methods are available for determining soil pH. These methods vary in their accuracy, cost, and ease of use. The best method for you will depend on your specific needs and resources.
- Laboratory Tests: These are generally the most accurate and detailed method. A soil sample is sent to a laboratory for analysis. Results typically include not only pH but also other crucial soil properties. This approach offers comprehensive data and detailed analysis.
- Home Test Kits: These kits provide a relatively quick and inexpensive way to test soil pH at home. They typically involve dipping a test strip or reagent into a soil sample and comparing the results to a color chart. These kits are convenient but may not be as precise as laboratory tests.
- Digital Soil pH Meters: These electronic devices measure soil pH directly in the field. They often provide readings quickly and conveniently. Accuracy depends on the quality of the meter and the proper calibration procedures. Calibration is critical for obtaining reliable results.
Comparing Soil pH Testing Methods
The table below summarizes the key differences between the various methods of soil pH testing.
| Method | Accuracy | Cost | Ease of Use | Time Required |
|---|---|---|---|---|
| Laboratory Tests | High | Moderate to High | Low | Several Days |
| Home Test Kits | Moderate | Low | High | Minutes |
| Digital Soil pH Meters | Moderate to High | Moderate | Moderate | Minutes |
Causes of Acidic Soil
Unveiling the secrets behind acidic soil conditions is crucial for effective soil management and sustainable agriculture. Understanding the factors contributing to acidity allows farmers and gardeners to proactively address the issue and maintain healthy, productive land. This understanding is essential for preventing yield reductions and maintaining soil fertility.Soil acidity, a critical aspect of soil health, is influenced by a complex interplay of natural processes and human activities.
From natural geological formations to agricultural practices, a variety of factors play a role in determining the pH level of soil. Understanding these interconnected forces provides the groundwork for implementing strategies to mitigate the negative impacts of acidic soil.
Natural Processes of Soil Acidification
Natural processes are significant contributors to soil acidification. The weathering of rocks, a gradual but continuous process, releases minerals that can alter soil pH. Certain minerals, like those containing sulfur and aluminum, can contribute to the acidification of the soil when they dissolve in water. Additionally, the decomposition of organic matter, a fundamental process in soil ecosystems, can also release acidic compounds.
The type of vegetation present also plays a significant role. For example, pine forests often contribute to acidic soil conditions due to the acidic nature of their needle litter.
Human Activities and Soil Acidity
Human activities significantly impact soil acidity. The widespread use of nitrogen-based fertilizers, while boosting crop yields, often leads to increased acidity. These fertilizers, particularly ammonium-based ones, release hydrogen ions into the soil as they break down. Furthermore, the application of sulfur-containing fertilizers and pesticides can also contribute to the problem. Industrial emissions and air pollution can deposit acidic compounds into the soil, further lowering its pH.
Influence of Environmental Factors
Rainfall patterns and irrigation practices are crucial factors affecting soil pH. Acid rain, a common environmental concern, directly contributes to soil acidification. The higher the acidity of rainwater, the more significant its impact on soil pH. Irrigation water, depending on its source and mineral content, can also influence soil pH. Excessive irrigation with water containing high levels of certain minerals can lead to increased soil salinity, impacting pH levels and overall soil health.
Agricultural Practices and Soil pH
Specific agricultural practices can have profound effects on soil pH. For example, the repeated application of acidifying fertilizers can lead to a significant decrease in pH over time. Crop choices also play a role. Certain crops, like legumes, can influence soil pH through their nutrient uptake and root exudates. Furthermore, the removal of soil through harvesting can cause changes in soil composition and consequently impact pH levels.
For instance, intensive cropping systems with little to no soil cover may experience more soil erosion and nutrient loss, influencing pH changes.
Correlation Between Factors and Soil Acidity
| Factor | Effect on Soil Acidity | Examples |
|---|---|---|
| Rock weathering | Can release acidic minerals | Granite weathering, releasing aluminum |
| Organic matter decomposition | Releases acidic compounds | Decomposition of plant residues |
| Nitrogen-based fertilizers | Increase acidity | Ammonium nitrate |
| Sulfur-containing fertilizers | Increase acidity | Sulfate fertilizers |
| Acid rain | Directly contributes to soil acidification | Increased rainfall acidity |
| Irrigation water | Can influence pH based on mineral content | Water with high calcium content, or high aluminum content |
| Crop choices | Can influence pH through nutrient uptake and root exudates | Legumes, pine trees |
| Agricultural practices (intensive cropping) | May increase soil erosion and nutrient loss | Monoculture cropping systems |
Impacts of Acidic Soil on Plants
Acidic soil, with its low pH, can significantly affect plant growth and health. This impact stems from the altered availability of essential nutrients, the potential for toxic elements to become mobile, and the disruption of beneficial soil organisms. Understanding these effects is crucial for successful gardening and agriculture in regions prone to acidic soil conditions.Acidic soil conditions often create a hostile environment for plant growth, leading to nutrient deficiencies and reduced overall vigor.
The specific effects can vary considerably depending on the type of plant, the severity of the acidity, and other environmental factors. Different plant species have varying tolerances to acidic soil, with some thriving in these conditions and others struggling to survive.
Effects on Plant Growth and Health
Acidic soil can impede nutrient uptake, leading to deficiencies in essential elements like nitrogen, phosphorus, and potassium. These deficiencies can manifest as stunted growth, yellowing leaves, and reduced yields. Furthermore, the altered soil chemistry can also increase the mobility of potentially toxic elements like aluminum, which can harm plant roots and overall health.
Responses of Different Plant Species
Different plant species have varying tolerances to acidic soil conditions. Some plants, like blueberries and azaleas, thrive in acidic environments. Others, such as many grasses and legumes, prefer neutral or slightly alkaline conditions. The specific adaptations of each plant species determine its ability to cope with the challenges posed by acidic soil.
Understanding acidic soil pH, or p2 as it’s sometimes called, is key for gardeners. Knowing the acidity level helps you choose the right plants for your garden, especially if you’re looking for plants that survive winter outside. Plants that survive winter outside often thrive in specific soil conditions, and understanding acidity is part of that. Ultimately, knowing your soil’s p2 is vital for successful gardening.
Nutritional Deficiencies in Acidic Soil
Plants grown in acidic soil often experience nutritional deficiencies. Aluminum toxicity, for instance, can impede root development, hindering nutrient uptake. Iron deficiency, while not directly caused by acidity, can become exacerbated in acidic soil. This leads to symptoms like chlorosis, a yellowing of leaves. Other deficiencies, such as those of phosphorus, calcium, and magnesium, can also be observed.
The extent of these deficiencies is directly related to the severity of the acidity.
Impact on Nutrient Availability and Uptake
Acidic soil alters the chemical environment, influencing the availability and uptake of essential nutrients. Essential nutrients like phosphorus and calcium become less available for plant absorption, leading to deficiencies. On the other hand, certain micronutrients, like iron, can become more soluble and potentially toxic in high concentrations. This imbalanced nutrient environment stresses plant growth and can result in diminished yield.
Comparison of Effects on Different Plant Types
The impact of acidic soil varies significantly between plant types. Acid-loving plants, like blueberries, are adapted to extract nutrients efficiently from acidic soil. However, plants like roses, preferring neutral soil, may suffer stunted growth or exhibit nutrient deficiencies in acidic conditions. Acidic soil can be detrimental to plant health, particularly if the acidity level is extremely high.
Summary Table of Impacts
| Plant Type | Response to Acidic Soil | Common Symptoms |
|---|---|---|
| Blueberries | Thrive in acidic conditions | Healthy growth, abundant fruit |
| Roses | Unfavorable conditions | Stunted growth, nutrient deficiencies, chlorosis |
| Grasses | Tolerant but may show signs of stress | Reduced vigor, yellowing leaves, stunted growth |
| Legumes | Prefer neutral or slightly alkaline | Reduced growth, poor flowering, potential root damage |
Soil Acidification Mitigation Strategies: What Is Acidic Soil P2
Acidic soil can significantly hinder plant growth and overall soil health. Fortunately, several effective strategies can mitigate the negative impacts of acidity and restore soil fertility. Understanding these methods is crucial for maintaining productive and thriving gardens, farms, and landscapes.A crucial step in managing acidic soil is understanding the specific needs of your soil type. Different soil types react differently to amendments, and a tailored approach ensures optimal results.
Knowing the type of lime to use, the appropriate application method, and the optimal time for application is essential to maximizing the effectiveness of soil amendments. By understanding these factors, you can effectively counteract the detrimental effects of acidic soil and promote a healthier environment for your plants.
Acidic soil pH2 is a tricky thing to understand, isn’t it? It’s all about the balance of elements in the soil, affecting plant growth. Speaking of things that aren’t quite right, have you ever looked at your bathroom and thought, “Ugh, what is this monstrosity?” Things like clashing tile colors or weird fixtures really bother designers, like in this article about things in your bathroom that give designers the ick.
Ultimately, getting the right pH level in your soil, just like getting the right look in your bathroom, takes some consideration. Understanding acidic soil pH2 is key to successful gardening.
Lime and Other Amendments for Adjusting Soil pH
Lime is the most common and effective amendment for raising soil pH. Different types of lime are available, each with varying effectiveness depending on the soil’s composition. The choice of lime depends on the specific needs of the soil and the desired pH level. Lime reacts with soil components to neutralize acidity, creating a more favorable environment for plant growth.
Process of Adding Lime to Soil
Adding lime to the soil is a crucial step in neutralizing acidity. Proper application ensures that the lime effectively reaches the soil particles, facilitating the pH adjustment process. This involves considering factors like soil type, lime type, and desired pH level.
Step-by-Step Procedure for Liming Acidic Soil
- Assess Soil pH: Determine the current pH level of your soil using a reliable soil testing kit. This crucial initial step helps you tailor the liming strategy for optimal results.
- Calculate Lime Requirements: Based on the soil test results and desired pH level, calculate the precise amount of lime needed. Local agricultural extension offices or soil testing labs can provide more precise calculations based on specific soil types.
- Choose Appropriate Lime Type: Select the lime type (e.g., agricultural lime, hydrated lime, dolomite lime) that best suits your soil type and desired pH level. Consider the cost-effectiveness and availability of different types.
- Thorough Mixing: Spread the lime evenly over the soil surface and thoroughly mix it into the top few inches of soil. Ensure complete integration to achieve uniform pH adjustment.
- Watering: Water the soil thoroughly after applying the lime. This helps dissolve the lime and facilitate its reaction with soil components.
- Monitoring and Retesting: Monitor the soil pH periodically to ensure the desired pH level is achieved. Repeat the liming process as needed based on retesting results.
Types of Lime Suitable for Different Soil Types
Different types of lime are more effective in different soil types. Agricultural lime, often made from limestone, is a common choice. Hydrated lime is another option, but it may be more reactive and require less application. Dolomite lime, derived from dolomite stone, contains magnesium and can be beneficial for soil deficient in magnesium. Understanding the specific composition of your soil helps determine the best lime type for your needs.
Alternative Soil Amendment Strategies for Reducing Acidity
Besides lime, other amendments can help reduce soil acidity. These include adding organic matter like compost, manure, or leaf litter. These materials increase soil organic matter, which helps buffer against pH fluctuations. Using sulfur is another option but is used to lower the pH, and is less common than increasing the pH with lime.
Table of Soil Amendments and Effectiveness
| Amendment | Effectiveness | Considerations |
|---|---|---|
| Lime (Agricultural, Dolomite, Hydrated) | High | Effective for raising pH, cost varies by type |
| Compost | Moderate | Improves soil structure, adds organic matter |
| Manure | Moderate | Adds nutrients and organic matter, potential for pathogens |
| Sulfur | Moderate to High (depending on soil type) | Effective for lowering pH, potential for environmental concerns |
Acidic Soil Management Practices
Maintaining healthy soil pH is crucial for successful gardening and agriculture. Understanding the intricacies of acidic soil management allows gardeners and farmers to create environments where plants thrive. This involves proactive measures to monitor and adjust soil conditions, ensuring optimal growth and yield.
Soil Testing and Monitoring, What is acidic soil p2
Regular soil testing is essential for understanding the current pH levels and nutrient status of the soil. This information is vital for developing targeted strategies for maintaining a healthy soil environment. By regularly analyzing soil samples, farmers and gardeners can proactively address any issues that might impact plant health. This proactive approach helps prevent potential problems before they significantly affect plant growth and yield.
Importance of Regular Soil Testing
Regular soil testing provides a snapshot of the soil’s current condition, enabling informed decisions regarding pH adjustment. This critical data allows for precise management of soil pH, optimizing nutrient availability and plant growth. Regular testing helps track the effectiveness of implemented management practices and facilitates adjustments to maintain ideal pH levels. For example, a farmer might notice that a specific field’s pH is consistently dropping, prompting them to implement strategies to counteract this trend.
Benefits of Soil Testing for Plant Health Management
Soil testing provides valuable insights into the nutrient levels and pH of the soil, which directly impacts plant health. Understanding the soil’s composition allows for targeted fertilization and pH adjustments, promoting robust plant growth. By identifying deficiencies or imbalances in nutrients, soil testing guides the application of appropriate fertilizers, ensuring that plants receive the necessary elements for optimal development.
This proactive approach minimizes the risk of nutrient deficiencies or toxicities, thus preventing stunted growth and maximizing yields.
Maintaining and Adjusting Soil pH Over Time
Maintaining optimal soil pH over time requires ongoing monitoring and adjustments. Understanding the factors that influence pH, such as rainfall, fertilizer use, and crop choices, is crucial for successful long-term management. For example, high rainfall can lead to leaching of alkaline elements, thus lowering the pH. Implementing strategies like using appropriate fertilizers and incorporating organic matter can help maintain a healthy balance.
Careful monitoring allows for timely adjustments to maintain the desired pH range for specific plant requirements.
Role of Crop Rotation in Managing Acidic Soil
Crop rotation plays a significant role in managing acidic soil by influencing soil pH over time. Different crops have varying nutrient requirements and impact on soil acidity. Certain crops can help restore the balance in acidic soil. For instance, legumes can enhance nitrogen content in the soil, improving its fertility and, consequently, its pH.
Influence of Different Cropping Systems on Soil pH
Different cropping systems have different effects on soil pH. Monoculture, where a single crop is consistently grown, can deplete specific nutrients and lead to pH imbalances. In contrast, diverse cropping systems, including rotation with legumes and cover crops, can help maintain a more stable and balanced pH level. For example, continuous cultivation of corn can deplete soil nutrients and potentially lead to increased acidity, whereas a rotation with soybeans, which fix nitrogen, can contribute to soil improvement.
Soil Management Practices for Maintaining Optimal pH
| Management Practice | Description | Impact on Soil pH |
|---|---|---|
| Soil Testing | Regular analysis of soil samples to determine pH levels and nutrient content. | Provides baseline data for pH management strategies. |
| Liming | Adding alkaline materials (lime) to raise soil pH. | Increases soil pH, making it more suitable for certain plants. |
| Organic Matter Incorporation | Adding organic matter (compost, manure) to improve soil structure and nutrient content. | Can slightly buffer pH changes and improve nutrient availability. |
| Crop Rotation | Alternating different crops in a field to maintain soil fertility and reduce acidity. | Influences pH through the different nutrient requirements of various crops. |
| Cover Cropping | Planting cover crops between main crops to protect soil and improve its fertility. | Can contribute to soil health and slightly influence pH. |
| Fertilizer Management | Using fertilizers judiciously to avoid nutrient imbalances that may impact pH. | Improper fertilizer application can lead to pH fluctuations. |
Case Studies of Acidic Soil Management
Acidic soils, a common problem in various agricultural regions, can significantly impact crop yields and overall soil health. Understanding how different regions and farms have tackled this issue, and the long-term effects of their chosen strategies, provides valuable insights for future management approaches. This section will delve into specific case studies, highlighting successful strategies, and analyzing the factors influencing soil pH changes.
Successful Acidic Soil Management Practices
Effective management of acidic soils requires a multifaceted approach. Strategies often include the application of soil amendments, adjusting agricultural practices, and monitoring soil pH. The success of these strategies hinges on understanding the specific characteristics of the soil and the desired outcome.
- Liming: A cornerstone of acidic soil management, liming involves adding alkaline materials like limestone to neutralize the acidity. This process raises the pH, making essential nutrients more available to plants. The effectiveness of liming depends on the type and amount of limestone used, as well as the specific soil characteristics. For instance, dolomitic limestone, containing magnesium, provides additional nutrients beyond just calcium.
- Organic Matter Incorporation: Adding organic matter, such as compost or manure, improves soil structure and increases its buffering capacity. This buffering capacity helps the soil resist changes in pH, making it more resilient to acidification. The type and quantity of organic matter play a critical role in the effectiveness of this strategy. For example, the use of cover crops like alfalfa can contribute significantly to soil organic matter content.
- Crop Rotation: Strategic crop rotation can also influence soil pH. Certain crops, like legumes, can contribute to nitrogen fixation, which can slightly increase soil pH over time. This strategy helps maintain soil fertility and reduce the reliance on external inputs.
Case Study Examples and Analysis
Analyzing successful case studies provides practical insights into the effectiveness of various soil amendment strategies.
- Case Study 1: Northeastern US Cranberry Bogs: Cranberry farms often face acidic soil conditions due to the specific peat-based soil and harvesting practices. Successful management in these areas frequently involves liming to increase pH and maintain optimal growing conditions for cranberries. Monitoring soil pH is crucial in these cases as the peat soils have limited buffering capacity.
- Case Study 2: Southeast Asia Rice Paddies: Rice cultivation in Southeast Asia often leads to soil acidification due to the intensive nature of the farming cycle. In these cases, liming, along with organic matter additions and crop rotation, can be used to address the issue. Farmers in these regions are often challenged by limited resources and knowledge. Successful practices involve community-based extension programs to educate and guide farmers.
Monitoring pH Changes
Accurate monitoring of soil pH after implementing management strategies is crucial to evaluate their effectiveness. Regular soil testing, using appropriate methods for the specific soil type, is necessary to track pH changes. This data allows for adjustments in the application of amendments and ensures that the chosen strategies are achieving the desired outcome. Ideally, soil pH monitoring should be done before and after implementing management strategies to establish a baseline and track changes over time.
Understanding acidic soil pH, or p2 as it’s sometimes called, is crucial for any gardener, but especially for first-time homeowners tackling renovations. Learning about soil pH levels can help you determine what plants will thrive in your yard, which directly impacts your landscaping choices. For example, knowing how to maintain the right pH level for your plants is an important skill to have when you’re starting out on your home improvement journey.
Checking your soil’s pH regularly and understanding the effects of pH changes on plant growth is vital in the long run, and is an essential part of any first-time homeowner renovation project. Learning more about pH and soil types will help you make smart choices for your garden, which is something that you can find out more about in our complete guide on first time homeowner renovation lessons.
Ultimately, this knowledge will help you maintain a healthy, vibrant garden, ensuring that your plants flourish and your home looks its best. So, if you want to understand what acidic soil p2 truly means, keep learning!
Long-Term Effects of Soil Amendment Strategies
The long-term effects of soil amendment strategies depend on several factors, including the specific amendments used, the initial soil conditions, and the ongoing management practices. Strategies that focus on long-term soil health, such as incorporating organic matter and employing crop rotation, are more likely to lead to sustainable improvements in soil pH and fertility.
Comparison of Case Studies
| Case Study | Soil Type | Primary Management Strategy | Key Success Factors | Challenges |
|---|---|---|---|---|
| Northeastern US Cranberry Bogs | Peat-based | Liming | Regular monitoring, precise application of lime | Limited buffering capacity of peat soil |
| Southeast Asia Rice Paddies | Acidic alluvial | Liming, organic matter, crop rotation | Community-based extension programs | Limited resources, access to information |
Soil pH Monitoring and Maintenance
Maintaining the ideal pH level in your soil is crucial for a healthy garden or farm. A consistent check on soil pH allows for proactive adjustments, preventing issues like nutrient deficiencies and stunted plant growth. Knowing your soil’s acidity levels empowers you to tailor your gardening practices for optimal plant health and sustainable agriculture.Understanding soil pH is not just about numbers; it’s about understanding the intricate relationship between the soil, the plants, and the overall ecosystem.
The ideal pH level for most plants falls within a specific range, and deviations can lead to significant problems. Regular monitoring and adjustments ensure that your soil provides the necessary nutrients and environment for healthy plant development, contributing to a more resilient and productive agricultural system.
Importance of Consistent Soil pH Monitoring
Regular soil pH monitoring is essential for maintaining soil health. A consistent pH level is vital for the availability of nutrients to plants. Nutrient availability is highly dependent on pH; certain nutrients are more readily absorbed by plants at specific pH levels. Inconsistent pH levels can lead to nutrient deficiencies, even if the soil contains ample amounts of nutrients.
Monitoring soil pH helps identify potential issues early on, enabling corrective measures before they negatively impact plant growth.
Role of Soil pH in Overall Soil Health
Soil pH significantly impacts the overall health and structure of the soil. It influences the microbial activity, decomposition rates, and the availability of essential nutrients. A balanced pH level supports the beneficial soil organisms that are crucial for nutrient cycling and soil fertility. Optimizing soil pH promotes a thriving ecosystem within the soil, which in turn benefits the plants growing in it.
For example, a balanced pH level allows for the proper decomposition of organic matter, enriching the soil with essential nutrients.
Using Indicators to Determine Soil Acidity
Various indicators can help determine soil acidity. These indicators range from simple, at-home tests to more sophisticated laboratory methods. These tests provide valuable insights into the soil’s acidity level, allowing for informed decisions about necessary adjustments. Accurate measurements enable precise and effective soil management practices.
Maintaining Optimal Soil pH Over Time
Maintaining optimal soil pH over time requires a proactive approach. Regular testing and adjustments are essential. Amendments like lime (calcium carbonate) can be used to increase pH in acidic soils. Alternatively, sulfur can be added to lower pH in alkaline soils. Careful monitoring and periodic adjustments ensure the soil remains in the optimal range for your specific plants.
This proactive approach ensures sustained soil health and plant growth.
Significance of pH Maintenance for Sustainable Agriculture
Maintaining optimal soil pH is a critical aspect of sustainable agriculture. It promotes healthy plant growth and minimizes the need for synthetic fertilizers and pesticides. This proactive approach minimizes environmental impact by reducing the use of potentially harmful chemicals. By maintaining a balanced pH, the agricultural practices become more environmentally friendly, reducing the strain on natural resources and promoting long-term soil fertility.
Simple Method for Regular Soil pH Monitoring at Home
A simple method for regular soil pH monitoring at home involves using a soil testing kit. These kits are readily available and relatively inexpensive. The testing procedure is straightforward and usually involves mixing a soil sample with a specific solution and observing the color change in the resulting solution. The color change is correlated to a pH scale, providing a quick and accurate reading of the soil’s acidity.
This simple method provides valuable data for adjusting soil pH, ensuring optimal plant growth.
Indicators of Soil Acidity
- Litmus Paper: This is a simple and inexpensive method for preliminary soil pH testing. It works by changing color in response to the acidity or alkalinity of the soil. The color change can be compared to a pH scale to get an approximate measurement.
- pH Test Kits: These kits provide more precise measurements. They often involve mixing a soil sample with a solution and observing the resulting color change. The color change corresponds to a specific pH value.
- Laboratory Analysis: For more in-depth analysis and detailed reports, laboratory testing provides highly accurate pH readings and further data about soil composition.
| Indicator | Description | Use |
|---|---|---|
| Litmus Paper | Changes color in response to acidity/alkalinity | Preliminary pH testing |
| pH Test Kits | Solution and color change for precise measurements | Accurate pH determination |
| Laboratory Analysis | Detailed soil analysis including pH | Comprehensive soil report |
Ending Remarks
In conclusion, managing acidic soil involves a multifaceted approach. From understanding the causes and impacts on plants to implementing effective mitigation strategies and consistent monitoring, this comprehensive guide equips you with the knowledge to cultivate thriving gardens in various soil conditions. By understanding the science behind soil pH, we can work towards sustainable agricultural practices and healthy plant growth.